Introduction
Olfactory neuroblastoma (esthesioneuroblastoma) is a rare neuroendocrine malignancy originating from the olfactory epithelium, first described by Berger et al. in 1924.[1] Accounting for less than 3% of sinonasal tumors, it poses diagnostic challenges due to nonspecific symptoms and histologic resemblance to other small round blue cell tumors.[2] Typical presentations such as nasal obstruction, epistaxis, and anosmia often lead to misdiagnosis as benign conditions like nasal polyps or sinusitis.[3] Diagnosis relies on histopathology showing small round cells with salt-and-pepper chromatin and immunohistochemical positivity for synaptophysin, INSM1, and chromogranin.2 Advanced imaging with 68Ga-DOTATATE PET-CT has improved staging and localization by exploiting somatostatin receptor expression.[4] Endoscopic resection with or without adjuvant radiotherapy remains the preferred treatment for early-stage disease, offering excellent disease control and functional outcomes.[5]
Case presentation
A 66-year-old African female with no significant comorbidities presented with a two-year history of progressive left nasal obstruction, intermittent epistaxis, and post-nasal drip. She was initially diagnosed with a benign nasal polyp and underwent endoscopic excision in the left nasal fossa. Postoperative histopathology revealed Hyams Grade 2 olfactory neuroblastoma.
On nasal endoscopy, a fleshy mass was identified occupying the left middle meatus. The patient did not have any other significant systemic symptoms or relevant past medical history.
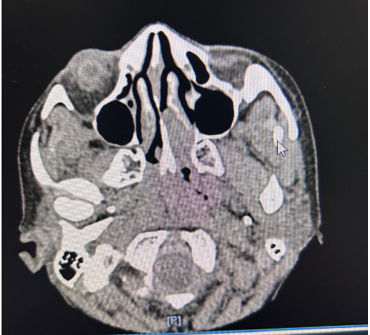
Cross-sectional CT imaging of the paranasal sinuses showed a soft tissue density lesion in the left nasal cavity centered in the middle meatus and olfactory cleft, without bony erosion or extension into the cribriform plate or orbit (Figure 1). Microscopically, the tumour showed small round blue cells with salt-and-pepper chromatin and Homer Wright pseudo-rosettes. Immunohistochemistry was positive for neuroendocrine markers (synaptophysin, chromogranin, INSM1) and negative for cytokeratin and S100, distinguishing it from sinonasal undifferentiated carcinoma or melanoma.
68Ga-DOTATATE PET-CT demonstrated moderate radiotracer uptake in the lesion without evidence of regional or distant metastases, confirming localized disease.
Kadish staging classified the tumour as Stage A (limited to the nasal cavity), and Hyams grading was Grade 2 (low-to-intermediate grade) with an anticipated 5-year survival of 80–90% but a moderate recurrence risk (10–30%).
The patient underwent excision and biopsy of the residual tumour with endoscopic resection. Intraoperative inspection revealed the tumour arose from the olfactory cleft without involving the cribriform plate, and histopathological examination confirmed negative margins. Adjuvant radiotherapy was delivered at 60 Gy in 30 fractions by intensity-modulated radiotherapy (IMRT). This schedule was chosen due to the 10–30% recurrence rate of Hyams Grade 2 tumours and the need to limit radiation dose to neighbouring optic structures which is an important benefit of IMRT
Post-treatment follow-up consisted of nasal endoscopy, showing healed mucosa without evidence of residual disease and Grade 1 mucositis which was managed symptomatically without treatment interruption. These findings support the effectiveness of combined endoscopic resection and IMRT for local control with preservation of tolerability.
Discussion
The diagnostic process for olfactory neuroblastoma is challenging, with the tumour often being masqueraded by benign polyps both on radiology and histology, thus requiring repeated biopsy with immunohistochemistry (INSM1) when there remains clinical suspicion. DOTATATE PET-CT has been found superior to FDG-PET because of its specificity for targeting somatostatin receptor expression, enabling more accurate tumor localization and staging. Therapeutically, endoscopic resection is now the norm for Kadish A/B tumours, with 5-year survival rates of 85–90%, and open craniofacial methods being reserved for advanced (Kadish C) disease. Radiotherapy protocols also continue to advance, with standard doses of 55–65 Gy now questioned by recent evidence that 50 Gy can be adequate for Hyams G1–2 tumours with clear margins; proton therapy further optimizes outcomes by decreasing orbital toxicity markedly. Molecular breakthroughs are transforming the landscape, with IDH2 inhibitors (e.g., enasidenib) under evaluation for IDH2-mutant disease and DLL3-targeted therapy (e.g., tarlatamab) being promising in relapse. These findings as a whole highlight the need for multidisciplinary collaboration to maximize diagnostic and treatment approaches to this rare cancer.
Endoscopic resection is currently the treatment of choice for Kadish A/B olfactory neuroblastoma, with high rates of disease control and less morbidity than with conventional open craniofacial techniques.5,6 Nicolai et al. have described five-year survival rates of up to 90% with endoscopic treatment, particularly when clear margins are obtained.[6] A meta-analysis by Rawal RB et al emphasized the importance of multidisciplinary collaboration and supported endoscopic techniques for early-stage sinonasal malignancies for its lower complication rates.[7] Craniofacial resection is typically reserved for advanced cases with intracranial involvement.[8]
Radiation therapy remains a mainstay of olfactory neuroblastoma management, particularly in intermediate or high-grade tumours or when surgical margins are uncertain. The NCCN guidelines recommend a dose of 55–65 Gy.[9] But newer evidence from current research indicates that reduced doses (about 50 Gy) can be adequate for low-grade (Hyams I–II) tumours with R0 resection, reducing radiation-associated toxicity. Proton beam therapy has been revealed to decrease optic and brain toxicity in skull base cancers compared to photon therapy, with Mehta et al. reporting decreased visual injury rates in olfactory neuroblastoma patients treated with protons.[10]
Olfactory neuroblastoma can simulate other small blue round cell tumours, thus histopathologic assessment is imperative. Typical characteristics involve small round cells with salt-and-pepper chromatin, fibrillary stroma, and Homer Wright rosettes.[11] Differentiation is supported by immunohistochemistry, wherein INSM1, synaptophysin, and chromogranin are positive, while cytokeratin and S100 are generally negative in Olfactory neuroblastoma.[12]
Chemotherapy has limited proven application in Olfactory neuroblastoma, especially in the early stages.[13]
The GETTEC group identified no survival benefit with chemotherapy in Kadish A/B patients. Neoadjuvant regimens are, however, being investigated for advanced, unresectable, or recurrent disease.[14] Molecular profiling has also identified new therapeutic targets. IDH2 mutations in olfactory neuroblastoma can be treated with anadenia, and DLL3-targeted therapy is under investigation in clinical trials for recurrent disease.[15]
Conclusion
This case highlights the diagnostic challenges of olfactory neuroblastoma, which often mimics benign nasal conditions, underscoring the importance of histopathological accuracy and multidisciplinary management. Treatment continues to evolve, with molecularly targeted therapies such as IDH2 inhibitors and DLL3-directed agents showing promise for recurrent or advanced disease. Ongoing research and prospective trials are essential to refine chemotherapy protocols and develop personalized treatment strategies for this rare malignancy.
Consent and ethics
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. The patient’s identity has been anonymized to protect confidentiality, and all clinical details have been presented without identifying information.
References
- Berger L, Luc R, Richard D. L’esthésioneuroépithéliome olfactif. Bull Assoc Fr Etude Cancer. 1924;13:410–21.
- Dulguerov P, Allal AS, Calcaterra TC. Esthesioneuroblastoma: a meta-analysis and review. Head Neck. 2001;23(9):502–5.
- Faragalla H, Weinreb I. Olfactory neuroblastoma: a review and update. Adv Anat Pathol. 2009;16(5):322–8.
- Broski SM, Hunt CH, Johnson GB, Morreale RF, Lowe VJ, et al. The value of 68Ga-DOTATATE PET/CT in esthesioneuroblastoma. Eur J Nucl Med Mol Imaging. 2016;43(3):418–28.
- Colevas AD, Cmelak AJ, Pfister DG, Spencer S, Adkins D, et al. NCCN Guidelines® Insights: Head and Neck Cancers, Version 2.2025. J Natl Compr Canc Netw. 2025 Feb;23(2):2-11. doi: 10.6004/jnccn.2025.0007. PMID: 39938471.
- Nicolai P, Battaglia P, Bignami M, Bolzoni Villaret A, Delù G, et al. Endoscopic surgery for malignant tumors of the sinonasal tract and adjacent skull base: a 10-year experience. Am J Rhinol. 2008;22(3):308–16.
- Rawal RB, Farzal Z, Federspiel JJ, Sreenath SB, Thorp BD, Zanation AM. Endoscopic resection of sinonasal malignancy: a systematic review and meta‐analysis. Otolaryngol Head Neck Surg 2016;155(3):376–386.
- Yuen AP, Fan YW, Fung CF, Hung KN. Endoscopic-assisted cranionasal resection of olfactory neuroblastoma. Head Neck. 2005 Jun;27(6):488-93. doi: 10.1002/hed.20193.
- Thawani R, Kim MS, Arastu A, Feng Z, West MT et al. The contemporary management of cancers of the sinonasal tract in adults. CA Cancer J Clin. 2023 Jan;73(1):72-112. doi: 10.3322/caac.21752. Epub 2022 Aug 2.
- Dagan R, Bryant C, Li Z, Yeung D, Justice J, Dzieglewiski P, Werning J, Fernandes R, Pirgousis P, Lanza DC, Morris CG, Mendenhall WM. Outcomes of Sinonasal Cancer Treated With Proton Therapy. Int J Radiat Oncol Biol Phys. 2016 May 1;95(1):377-385. doi: 10.1016/j.ijrobp.2016.02.019. Epub 2016 Feb 12.
- Miyaguchi M, Kitaoku S, Sakai S, Uda H. Clinical and histopathological studies of olfactory neuroblastoma. Auris Nasus Larynx. 1989;16(3):157-63. doi: 10.1016/s0385-8146(89)80013-6.
- Rooper LM, Bishop JA, Westra WH. INSM1 is a Sensitive and Specific Marker of Neuroendocrine Differentiation in Head and Neck Tumors. Am J Surg Pathol. 2018 May;42(5):665-671. doi: 10.1097/PAS.0000000000001037.
- Tosoni A, Di Nunno V, Gatto L, Corradi G, Bartolini S, Ranieri L, Franceschi E. Olfactory neuroblastoma: diagnosis, management, and current treatment options. Front Oncol. 2023 Oct 16;13:1242453. doi: 10.3389/fonc.2023.1242453.
- Halwani C, Ferchichi S, Messelmani M. Successful Management of Advanced Olfactory Esthesioneuroblastoma: A Case Report. Clin Case Rep. 2025 Oct 15;13(10):e71208. doi: 10.1002/ccr3.71208.
- Ghanem A, Finlay JB, Jang DW, Goldstein BJ, Abi Hachem R. Recent developments in olfactory neuroblastoma research. Curr Opin Otolaryngol Head Neck Surg. 2025 Feb 1;33(1):50-55. doi: 10.1097/MOO.0000000000001027.