Introduction
Thymoma is a rare epithelial malignancy that arises from the thymic epithelium. It accounts for less than 1% of all adult tumors. Despite its relatively indolent oncologic behavior, thymoma is commonly associated with immune dysregulation and a paraneoplastic autoimmune phenomenon [1]. The thymus plays a central role in T-cell maturation and immune tolerance and neoplastic transformation of thymic epithelium can result in profound disturbances of immune homeostasis. This can lead to manifestations such as autoimmunity, immunodeficiency or in some cases a combination of both [2].
Autoimmune manifestations associated with thymoma most commonly include myasthenia gravis but may also consist of pure red cell aplasia, autoimmune thyroid disease, inflammatory arthritis, dermatologic disorders and gastrointestinal involvement [3]. These manifestations may involve multiple organ systems simultaneously and can precede the diagnosis of thymoma. In many cases these manifestations are misdiagnosed initially as primary connective tissue diseases or systemic inflammatory disorders. Thymoma-associated multiorgan autoimmunity (TAMA) represents a rare and severe form of paraneoplastic autoimmunity which is characterized by inflammatory involvement of the skin, joints, gastrointestinal tract as well as endocrine organs [4].
Good’s syndrome is a distinct and uncommon immunodeficiency associated with thymoma. It is characterized by hypogammaglobulinemia, reduced or absent B cells, impaired cellular immunity and increased susceptibility to infections [5]. Patients typically present in middle adulthood with history of recurrent bacterial, viral or opportunistic infections. Chronic diarrhoea, mucocutaneous lesions and hematologic abnormalities are other common manifestations. Importantly immunodeficiency in Good’s syndrome often persists despite thymectomy thereby contributing to significant morbidity and mortality [6].
The coexistence of thymoma-associated multiorgan autoimmunity and Good’s syndrome is exceptionally rare and represents a complex correlation between autoimmunity and immunodeficiency [7]. Such presentations pose substantial diagnostic challenges due to their nonspecific clinical features. Awareness of these rare thymoma-related immune syndromes is essential for early diagnosis and appropriate management [8].
We describe a case of thymoma-associated multiorgan autoimmunity presenting with inflammatory polyarthritis, mucocutaneous lesions, adrenal insufficiency, and chronic diarrhoea, along with persistent hypogammaglobulinemia consistent with Good’s syndrome.
Case presentation
A 42-year-old male presented with a four-month history of intermittent high-grade fever with chills, chronic loose stools, progressive weight loss, joint pain, and skin changes. Loose stools occurred approximately four times per day, predominantly postprandial and nocturnal, without mucus, blood, or tenesmus. Joint pain mainly involved the large joints of the lower limbs and lumbosacral region, with morning stiffness sparing small joints. Progressive hyperpigmentation of the face, neck, and upper back, painful skin erosions over the gluteal region and elbows and mouth ulcers were reported.
On examination, he was febrile (102°F), tachycardic (110/min), and hypotensive (96/60 mmHg) with orthostatic drop. There was generalized hyperpigmentation, multiple oral ulcers with velvety plaques as well as inflammatory arthritis of knees, ankles and elbows. Skin erosions were present over the gluteal region and exfoliative lesions were seen over both elbows. Abdominal examination revealed diffuse tenderness with increased bowel sounds, without distension or organomegaly. Respiratory examination showed bilateral rhonchi with normal breath sounds. Musculoskeletal evaluation demonstrated restricted range of motion in involved joints, with tender joint count of 10 and swollen joint count of 6. In summary, the patient presented with a systemic inflammatory illness characterized by fever, inflammatory arthritis, mucocutaneous lesions, chronic diarrhoea, endocrine dysfunction, and weight loss (Figure 1).
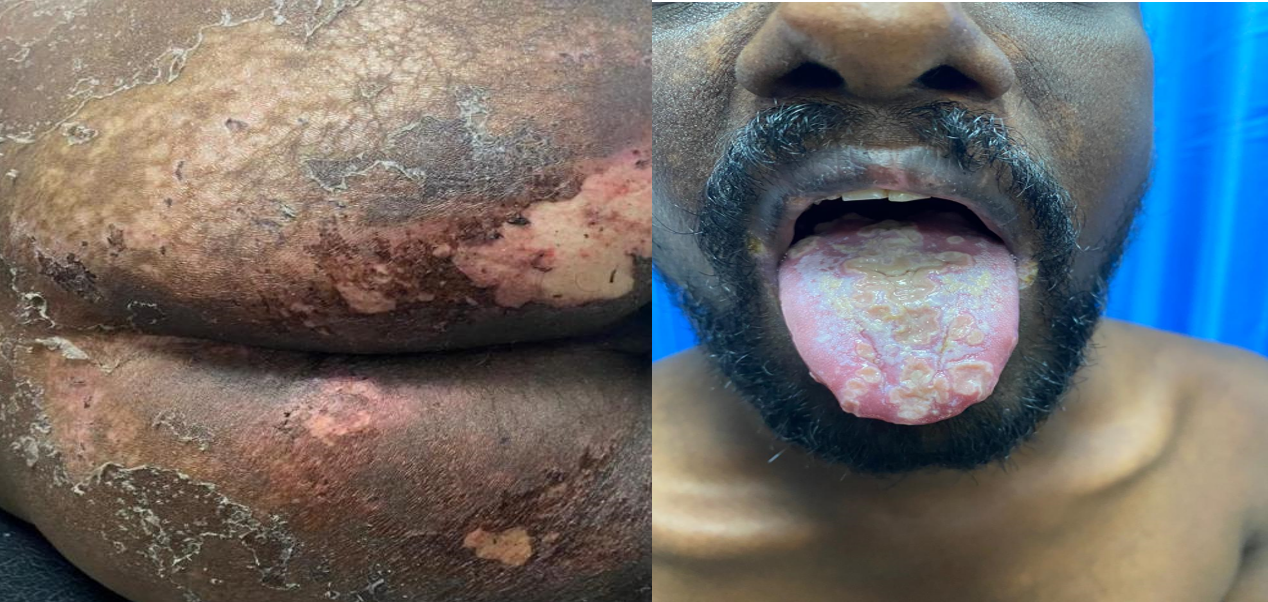
Based on the clinical features of chronic diarrhoea, systemic inflammation and extraintestinal manifestations, inflammatory bowel disease with associated extraintestinal features was initially considered. Post-infectious reactive arthritis and chronic inflammatory disorders ( systemic lupus erythematosus and Behçet’s disease) were included in the differential diagnoses given the presence of inflammatory arthritis and mucocutaneous lesions. Malabsorption syndrome was additionally considered in view of presence of chronic diarrhoea, weight loss and electrolyte disturbances.
Routine investigations showed neutrophilic leukocytosis and elevated inflammatory markers (ESR 46 mm/hr, CRP 114 mg/L). Serum sodium was 121 mEq/L and chloride 86 mEq/L. Stool analysis showed 2–5 pus cells/HPF and fungal elements without fat globules. Fecal calprotectin was elevated and Anti-Saccharomyces cerevisiae Antibodies (ASCA) was negative. Blood and stool cultures were sterile, and infectious disease panel was negative. Endoscopy showed Helicobacter pylori gastritis and colonoscopy was normal. Endocrine evaluation revealed low morning cortisol. ANA-IIF and anti-CCP were negative. Tzanck smear of exfoliative skin lesion showed no acantholytic or multinucleated giant cells. Ophthalmologic evaluation ruled out uveitis. Liver function testing showed hypogammaglobulinemia, prompting evaluation for immunodeficiency.
The patient was diagnosed with thymoma-associated multiorgan autoimmunity manifesting as adrenal insufficiency, inflammatory polyarthritis and mucocutaneous involvement. Additionally presence of persistent hypogammaglobulinemia with recurrent infections and chronic diarrhoea established the diagnosis of Good’s syndrome. The patient received sulfasalazine, mesalamine, H. pylori eradication therapy, corticosteroids, antidiarrheal therapy and multivitamins. Due to persistent diarrhoea and inflammatory symptoms, contrast-enhanced CT was performed revealing a heterogeneously enhancing mediastinal mass (Figure 2).
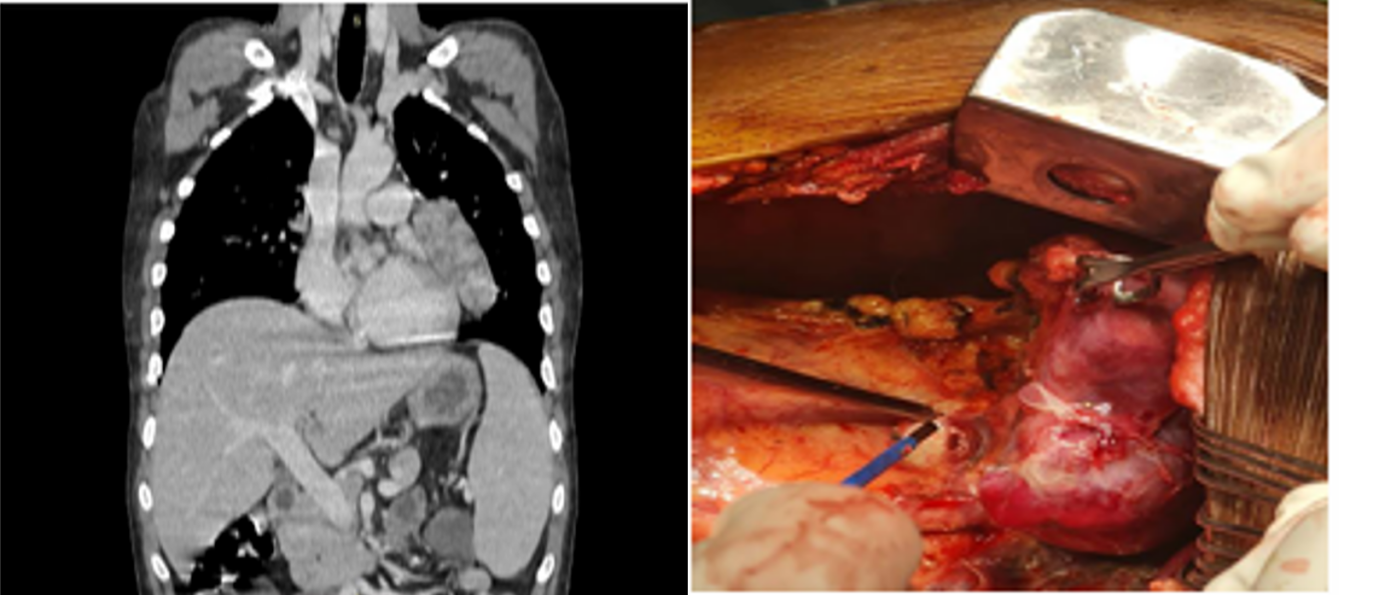
PET-CT confirmed a lobulated lesion in the left paracardiac and aortopulmonary region without metastasis.
He underwent surgical excision, and histopathology confirmed Type-A thymoma. Postoperatively, he developed neutropenic sepsis which responded to broad-spectrum antibiotics and granulocyte colony-stimulating factor. Following thymectomy, polyarthritis, fever, and mucocutaneous lesions improved and diarrhoea reduced significantly. Despite clinical improvement, persistent hypogammaglobulinemia and recurrent infections continued during follow-up. Immunoglobulin electrophoresis confirmed markedly reduced immunoglobulin levels, establishing Good’s syndrome.
Discussion
Thymoma-associated multiorgan autoimmunity is rare and may mimic connective tissue disorders or inflammatory bowel disease. The coexistence of Good’s syndrome further complicates immunological status due to combined cellular and humoral immunodeficiency [9]. Although thymoma is classically associated with myasthenia gravis and pure red cell aplasia, simultaneous inflammatory arthritis, mucocutaneous lesions, adrenal insufficiency, chronic diarrhoea, and hypogammaglobulinemia—as seen in this case—is unusual.
Improvement of systemic inflammation after thymectomy supports the autoimmune pathogenesis of TAMA, while persistent hypogammaglobulinemia and recurrent infections confirm the irreversible immunodeficiency of Good’s syndrome. Literature reports show that even after tumor removal, immune dysregulation frequently persists, predisposing patients to recurrent and severe infections [10].
This case highlights the need to consider thymoma in adults with unexplained systemic inflammation, chronic diarrhoea, mucocutaneous lesions, or recurrent infections. Early imaging and immunoglobulin profiling can facilitate timely diagnosis. Long-term management requires antimicrobial prophylaxis and immunoglobulin replacement.
Conclusion
This case describes an uncommon presentation of thymoma with multiorgan autoimmunity and concomitant Good’s syndrome. Persistent immunodeficiency despite thymectomy underscores the chronic nature of Good’s syndrome. Clinicians should suspect thymoma and associated immunodeficiency in adults with unexplained chronic diarrhoea, recurrent infections, mucocutaneous lesions, and polyarthritis. Early diagnosis and multidisciplinary management are essential for improving outcomes.
Consent and ethics
Ethics Approval and consent was waived since no identifiable information is published and all patient data was anonymised.
References
- Fend F, Dogan A, Cook JR. Plasma cell neoplasms and related entities—evolution in diagnosis and classification. Virchows Arch. 2023 Jan;482(1):163-177. doi: 10.1007/s00428-022-03431-3.
- Hatipoğlu U, Seyhan M, Ulas T, Dal MS, Altuntaş F. Solitary Plasmacytomas: Current Status in 2025. Hematol Rep. 2025 Jun 30;17(4):32. doi: 10.3390/hematolrep17040032.
- Mogoantă CA, Sarafoleanu C, Osman A, Enache I, Tarabichi S, et al. Extramedullary Plasmacytomas of the Nasal Cavity: Case-Based Perspectives into Optimizing the Diagnostic Differentiation from Inflammatory Polyps. Medicina (Kaunas). 2025 Aug 1;61(8):1406. doi: 10.3390/medicina61081406.
- Lu T, Pu H, Zhao G. Primary pancreatic plasmacytoma: a rare case report. BMC Gastroenterol. 2017 Dec 20;17(1):167. doi: 10.1186/s12876-017-0729-z.
- Rizk RC, Weisberg EM, Fishman EK. Solitary plasmacytoma of the pancreas: A rare case report. Radiol Case Rep. 2024 Feb 17;19(5):1806-1809. doi: 10.1016/j.radcr.2024.01.065.
- Leake PA, Coard KC, Plummer JM. Extramedullary plasmacytoma of the pancreas as an uncommon cause of obstructive jaundice: a case report. J Med Case Rep. 2009 Aug 6;3:8785. doi: 10.4076/1752-1947-3-8785.
- Dass J, Arava S, Mishra PC, Dinda AK, Pati HP. Role of CD138, CD56, and light chain immunohistochemistry in suspected and diagnosed plasma cell myeloma: A prospective study. South Asian J Cancer. 2019 Jan-Mar;8(1):60-64. doi: 10.4103/sajc.sajc_64_17.
- Barbosa P, de Diego C, Anaya J, Busso C, et al. Primary extramedullary plasmacytoma: a rare case presentation. An Bras Dermatol. 2025 Jul-Aug;100(4):501137. doi: 10.1016/j.abd.2025.501137.
- Caers J, Paiva B, Zamagni E, Leleu X, Bladé J, et al. Diagnosis, treatment, and response assessment in solitary plasmacytoma: updated recommendations from a European Expert Panel. J Hematol Oncol. 2018 Jan 16;11(1):10. doi: 10.1186/s13045-017-0549-1.
- Nashed B, Khan A, Issa M, Kohler L, Barawi M. Pancreatic Plasmacytoma: A Case of Recurrent Disease. Cureus. 2022 Jul 1;14(7):e26502. doi: 10.7759/cureus.26502.