Introduction
Pancreatic pseudocysts are localized collections of pancreatic fluid rich in enzymes, blood and necrotic tissue.[1] They are typically encapsulated by a fibrous, non-epithelial wall. They usually arise as sequelae of acute or chronic pancreatitis, pancreatic trauma or ductal disruptions and are most often located in the lesser sac or adjacent peripancreatic regions. The global incidence of pseudocysts in patients with chronic pancreatitis ranges between 20% to 40% with males in the third to fifth decade of life being most commonly affected.[2] These pseudocysts are often seen in association with alcohol-induced chronic pancreatitis.[3] Aberrant locations such as intraparenchymal, intra-mesenteric, mediastinal, or even prehepatic sites are rarely reported. Among these, prehepatic pancreatic pseudocysts—accumulating anterior to the liver in the potential space of the lesser omentum or falciform ligament—represent an exceptional and rare anatomical variant.
In typical surgical practice, pancreatic pseudocysts often present with persistent abdominal pain, a palpable mass and early satiety which is often preceded by an episode of pancreatitis. However, in atypical locations such as prehepatic space, pseudocyst may present with vague upper abdominal swelling or signs mimicking hepatic or subphrenic pathologies. As such, accurate anatomical localization becomes crucial to differentiate pseudocysts from hepatic cysts, subphrenic abscesses, echinococcal cysts and neoplastic cystic lesions of the liver.[4]
The diagnosis is especially challenging in rural tertiary care centers where reliance is often placed on ultrasonography and contrast-enhanced computed tomography (CECT) given the limited availability of endoscopic ultrasound (EUS) or magnetic resonance cholangiopancreatography (MRCP).[5] Radiological imaging remains the cornerstone in differentiating these entities and in defining the extent and communications of the cyst with the pancreatic ductal system.[6] Management strategies for pancreatic pseudocysts are guided by symptomatology, cyst size, duration, presence of complications (such as infection, hemorrhage, or rupture) and presence of communication with the pancreatic duct.[7] Conventional peripancreatic pseudocysts can often be managed conservatively or via image-guided drainage or endoscopic procedures. However, pseudocysts in unusual locations such as the prehepatic space frequently necessitate surgical intervention due to their size, anatomical complexity or due to failure of non-operative measures.[8]
Cystogastrostomy, either open or laparoscopic, remains the mainstay of treatment for large, symptomatic pseudocysts that abut the stomach wall, especially in cases where endoscopic access is limited or contraindicated. Intraoperatively these pseudocysts may be found adherent to the hepatic surface or peritoneum thereby necessitating careful dissection to avoid iatrogenic injury. Postoperative outcomes are generally favorable with appropriate surgical planning and follow-up.[9]
Although several case reports and series have documented atypical presentations of pancreatic pseudocysts reports of prehepatic pseudocysts remain exceedingly rare. The pathophysiology of such localization is attributed to posterior leakage of pancreatic enzymes traversing anatomical planes such as the hepato-gastric ligament or through the foramen of Winslow into the anterior subhepatic space. These anatomical routes are not commonly involved in standard pseudocyst formation making the prehepatic pseudocysts an exceptional phenomenon.[10] The clinical relevance of recognizing such a presentation lies in preventing misdiagnosis and inappropriate interventions for presumed hepatic or subdiaphragmatic masses.
The present case aims to analyse the clinical presentation, radiological features, surgical management and postoperative outcome of a prehepatic pancreatic pseudocyst in a 35-yearold male treated at a tertiary care center in rural central India.
Case presentation
A 35-year-old male presented to department of surgery with a progressively enlarging upper abdominal lump for 1 month. The swelling was insidious in onset, gradually progressing and was confined to the upper abdomen. He also reported dull aching epigastric pain for the same duration. The pain was described as deep visceral, non-radiating and was of moderate
intensity. There was no history of fever, abdominal trauma, jaundice or radiating pain. Similarly, there were no bowel or bladder complaints. On examination, the patient was hemodynamically stable with a pulse rate of 86/min, blood pressure of 130/80 mmHg. Oxygen saturation was found to be 99% on room air. He appeared thin with a body mass index of 17.3 kg/m² and was having pallor. The remainder of the systemic examination including respiratory and cardiovascular examination was unremarkable. Abdominal inspection revealed a visible
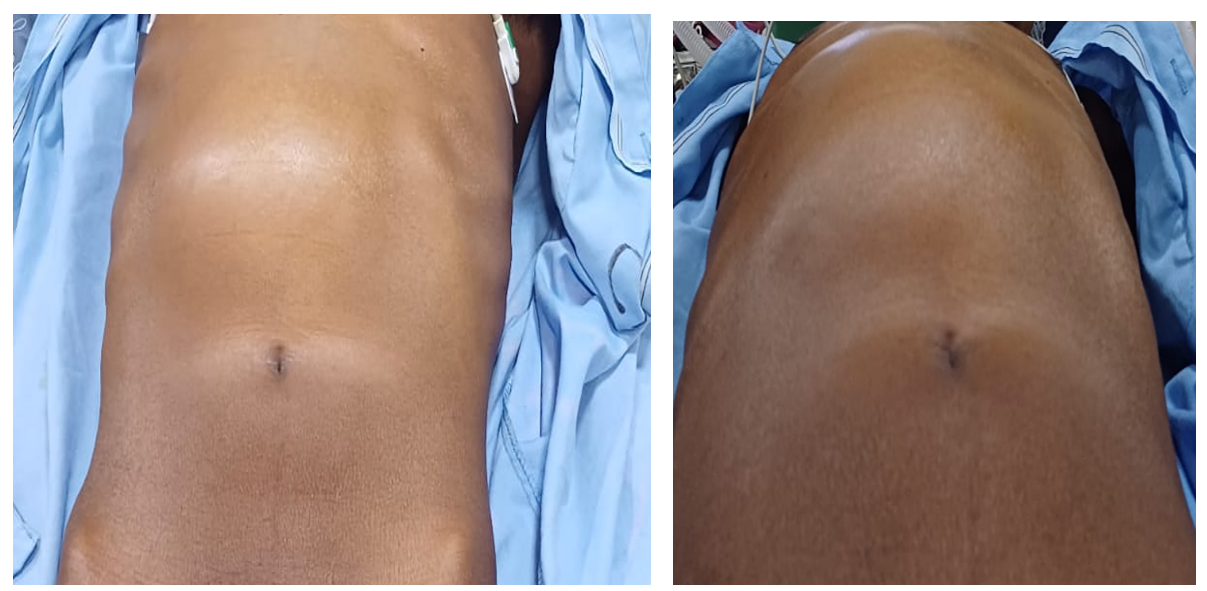
swelling in the upper abdomen which was extending from the epigastrium to the left hypochondrium. It measured approximately 15×15 cm. Palpation confirmed a solitary, globular, smooth, firm-to-hard and non-tender epigastric mass extending into the left hypochondrium. There was no cough impulse and no appreciable mobility was demonstrated. Percussion was dull over the mass. Bowel sounds were normal (Figure 1).
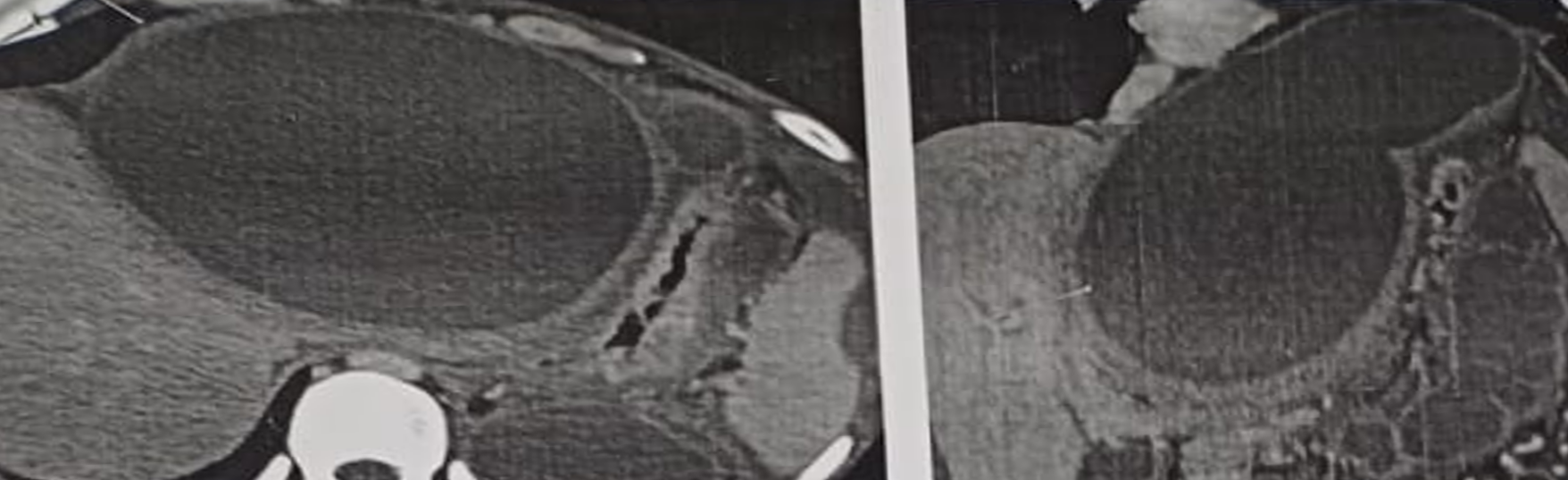
contrast-enhanced computed tomography of the abdomen was done which demonstrated a large thick-walled peripancreatic collection with extension into the perihepatic region and further into the perisplenic and gastrosplenic regions producing significant mass effect on the stomach. The posterior wall thickness of the collection measured approximately 5 mm. The collection extended up to the pancreatic tail and was closely related to a dilated pancreatic duct with suspected ductal communication. Additional thickwalled, intercommunicating collections were noted in the lesser sac and perinephric region causing mass effect on the left kidney and adrenal gland. Imaging also showed features of chronic calcific pancreatitis including an atrophic pancreas, a prominent pancreatic duct and intraparenchymal calcifications. Chronic splenic vein thrombosis was identified with multiple perisplenic, perigastric, peripancreatic, and fundal collaterals. The modified computed tomography severity index was 6/10, consistent with moderate severity (Figure 2).
In view of the large symptomatic collection with significant mass effect and the radiologic impression of a mature pseudocyst in the background of chronic pancreatitis surgical intervention was undertaken. Intraoperatively, a large epigastric cystic mass was identified with dense adhesions between the cyst the peritoneum and the left lobe of the liver. The stomach was displaced leftwards. A cystogastrostomy was performed, and approximately 2.5 litres of thick cyst fluid was drained. The cystogastrostomy anastomosis was done using a stapling technique. (Figure 3).
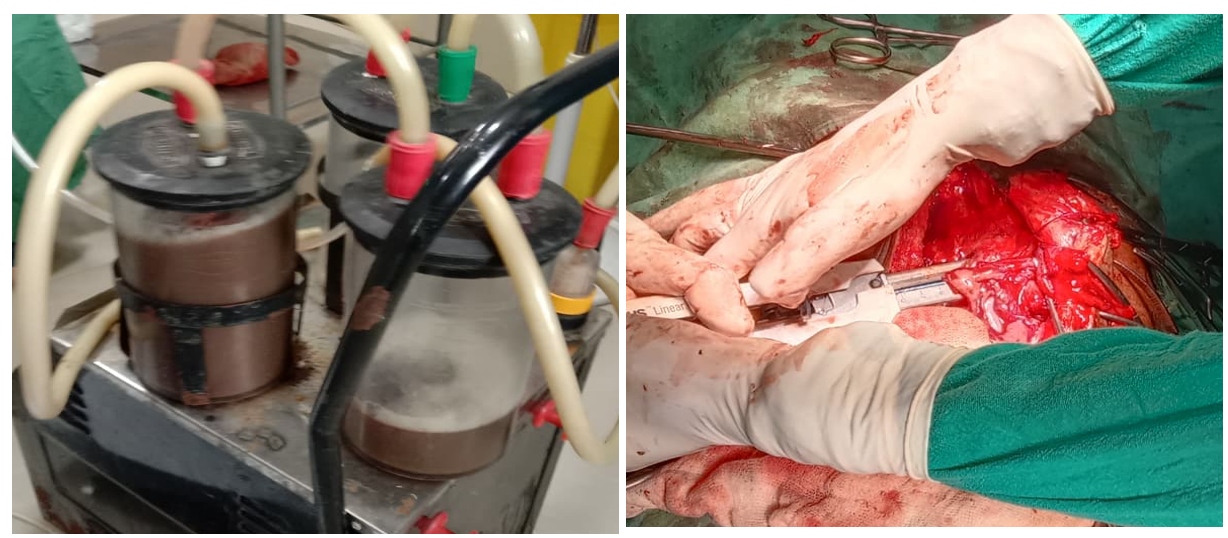
The postoperative course was uneventful without major complications. The patient improved clinically and was discharged with advice for follow-up.
Discussion
Pancreatic pseudocysts are well-recognized complications of acute and chronic pancreatitis forming as encapsulated pancreatic secretions devoid of epithelial lining and typically confined to peripancreatic spaces. Most commonly, pseudocysts occur in the lesser sac, retroperitoneum or adjacent to the pancreatic head and body where fluid collects along natural fascial planes. These locations account for the majority of clinically encountered pseudocysts. However, the literature also reveals a spectrum of unusual pseudocyst localizations reporting extensions into the mediastinum, perinephric spaces, iliopsoas muscle and even the pleural cavity. These unusual locations underscore the ability of pancreatic secretions to extend into fascial planes far away from the original site of inflammation. These atypical pseudocyst presentations that may (including the prehepatic location observed in our case) are noteworthy because they may mimic unrelated pathologies such as hepatic cysts, subphrenic collection or subhepatic mass lesions.[11] In our patient, contrast-enhanced CT revealed a large thick-walled collection extending anterior to the liver, communicating with a dilated pancreatic duct and consistent with a mature pseudocyst in the setting of chronic pancreatitis. Accurate imaging analysis was critical in distinguishing this lesion from primary hepatic or subdiaphragmatic pathology.
Unusual pseudocyst localizations have been documented in the literature. Khaladkar et al described extensions of pseudocysts into muscular and extraperitoneal planes such as the psoas, intercostal spaces and lumbar regions.[12] These cases indicated the potential for pancreatic fluid to traverse anatomically distant fascial spaces. Similarly, Tomar et al reported multiple intrahepatic pseudocysts thereby highlighting a highly uncommon manifestation of pseudocyst formation where collections extend into hepatic parenchyma via the hepatoduodenal or hepatogastric ligaments.[13] The review by Demeusy et al analysed literature on intrahepatic pancreatic pseudocysts (IHPPs).[14] This review noted their rarity and the diagnostic challenges posed by their imaging appearance which often is reported to overlap with cystic hepatic lesions. These observations support the concept that pancreatic secretions, particularly in chronic pancreatitis, can travel along less familiar anatomical pathways including peritoneal ligaments or retroperitoneal planes to produce collections in atypical sites.
The pathophysiology of unusual pseudocyst formation reflects the interplay between ductal disruption, increased intraductal pressure as well as communication with surrounding structures. In chronic pancreatitis, ongoing ductal obstruction and repeated bouts of inflammation promote leakage of pancreatic secretions which may dissect along connective tissue planes. Baydar et al described various mechanisms by which pancreatic fluid may pass posterior to the hepatoduodenal ligament and along the hepatogastric ligament.[15] This consequently can erode hepatic parenchyma and result in intrahepatic pseudocyst formation. Similarly, Topno N emphasized that hepatic involvement is a recognized but rare extension of pseudocyst pathology.[16] Very few cases of intrahepatic pseudocyst have been reported in the literature to date. The relative scarcity of such pseudocysts found in unusual locations in published reports underscores the importance of expanding clinical awareness of such pseudocyst variants.
Management strategies for pancreatic pseudocysts are guided by symptom severity, cyst size and maturity. Tan et al's review on pseudocyst management highlights spectrum of therapeutic approaches ranging from conservative and percutaneous drainage to surgical interventions. As per this review large size of the pseudocysts, symptomatic collections or those with ductal communication often necessitate drainage.[17] The choice between percutaneous radiologic, endoscopic or surgical drainage is dictated by anatomical factors and institutional expertise. Pseudocysts adjacent to the stomach or duodenum are suitable for cystogastrostomy. Suggs et al demonstrated that surgical cystenterostomy (including cystogastrostomy) remains a highly effective option with favorable morbidity and mortality when compared with other techniques.[18] In our case an open cystogastrostomy yielded satisfactory drainage of approximately 2.5 Liters of thick cystic fluid and resulted in an uneventful postoperative course. Although less invasive options such as endoscopic ultrasound (EUS)-guided drainage are increasingly utilized when feasible.
While endoscopic drainage (including EUS guidance) is often first-line for accessible pseudocysts and has demonstrated high success rates with lower invasiveness, it may be restricted by cyst proximity to the gastrointestinal lumen or by complex adhesions, as encountered in our patient Chahal P et al and Bhasin et al reported successful endoscopic transpapillary management of intrahepatic pancreatic pseudocysts in select cases.[19] Endoscopic options can be effective in experienced hands even in atypical locations. However, for large symptomatic collections surgical intervention remains the preferred definitive management strategy. In the context of resource-limited settings where availability of endoscopic modalities may be restricted, surgical cystogastrostomy provides a reliable alternative with excellent clinical outcomes.[20]
In summary our case exemplifies the diagnostic and therapeutic challenges associated with an extremely rare prehepatic pancreatic pseudocyst. Comparable case reports highlight that pseudocysts can present in a variety of unusual anatomical sites, demanding a high index of suspicion and thorough radiological investigations.
Conclusion
Prehepatic pancreatic pseudocyst is an extremely rare manifestation of chronic pancreatitis that may losely mimic hepatic or subphrenic pathology. Appropriate imaging techniques such as computed tomography or Magnetic Resonance imaging are essential for appropriate diagnosis and surgical planning. In resource-limited settings early diagnosis and timely surgical intervention is known to be associated with favorable outcomes. Increased awareness of atypical locations of pseudocyst is essential to prevent misdiagnosis and ensure appropriate management.
Consent and ethics
Written informed consent was obtained from the patient for publication.
References
- Koo JG, Liau MYQ, Kryvoruchko IA, Habeeb TA, Chia C, Shelat VG. Pancreatic pseudocyst: The past, the present, and the future. World J Gastrointest Surg. 2024 Jul 27;16(7):1986-2002. doi: 10.4240/wjgs.v16.i7.1986. PMID: 39087130; PMCID: PMC11287700.
- Byrne MF, Mitchell RM, Baillie J. Pancreatic Pseudocysts. Curr Treat Options Gastroenterol. 2002 Oct;5(5):331-338. doi: 10.1007/s11938-002-0021-2. PMID: 12207856.
- O'Malley VP, Cannon JP, Postier RG. Pancreatic pseudocysts: cause, therapy, and results. Am J Surg. 1985 Dec;150(6):680-2. doi: 10.1016/0002-9610(85)90407-6. PMID: 3907380.
- Mavilia MG, Pakala T, Molina M, Wu GY. Differentiating Cystic Liver Lesions: A Review of Imaging Modalities, Diagnosis and Management. J Clin Transl Hepatol. 2018 Jun 28;6(2):208-216. doi: 10.14218/JCTH.2017.00069. Epub 2018 Jan 5. PMID: 29951366; PMCID: PMC6018306.
- Podgurski L, Hou G, Shaffer K. CT Imaging of a Pancreatic Pseudocyst: Clinical and Anatomic Implications. Radiol Case Rep. 2015 Dec 7;2(4):107. doi: 10.2484/rcr.v2i4.107. PMID: 27303493; PMCID: PMC4895868.
- Kim YH, Saini S, Sahani D, Hahn PF, Mueller PR, Auh YH. Imaging diagnosis of cystic pancreatic lesions: pseudocyst versus nonpseudocyst. Radiographics. 2005 May-Jun;25(3):671-85. doi: 10.1148/rg.253045104. PMID: 15888617.
- Gurusamy KS, Pallari E, Hawkins N, Pereira SP, Davidson BR. Management strategies for pancreatic pseudocysts. Cochrane Database Syst Rev. 2016 Apr 14;4(4):CD011392. doi: 10.1002/14651858.CD011392.pub2. PMID: 27075711; PMCID: PMC6457582.
- Aghdassi AA, Mayerle J, Kraft M, Sielenkämper AW, Heidecke CD, Lerch MM. Pancreatic pseudocysts--when and how to treat? HPB (Oxford). 2006;8(6):432-41. doi: 10.1080/13651820600748012. PMID: 18333098; PMCID: PMC2020756.
- Kiviluoto T, Kivisaari L, Kivilaakso E, Lempinen M. Pseudocysts in chronic pancreatitis. Surgical results in 102 consecutive patients. Arch Surg. 1989 Feb;124(2):240-3. doi: 10.1001/archsurg.1989.01410020114019. PMID: 2916944.
- Kim HJ, Jun CH, Park CH, Cho CK. Intrahepatic Pancreatic Pseudocyst Complicated by Pancreatitis: A Case Report. Korean J Gastroenterol. 2017 Oct 25;70(4):202-207. doi: 10.4166/kjg.2017.70.4.202. PMID: 29060959.
- Shrestha G, Rajbhandari S, Karki B, Bashya B, Ghimire B. Intrahepatic pancreatic pseudocyst (wayward cyst): A rare presentation of traumatic pancreatitis. Int J Surg Case Rep. 2024 Jan;114:109125. doi: 10.1016/j.ijscr.2023.109125. Epub 2023 Dec 14. PMID: 38100928; PMCID: PMC10762353.
- Khaladkar SM, Kamal V, Kamal A, Shinde K, Suryawanshi P. Unusual locations of pancreatic pseudocysts: psoas, lumbar triangle and intercostal spaces. J Clin Imaging Sci. 2018;8:33. doi:10.4103/jcis.JCIS_54_18
- Tomar S, Ghasi RG, Agarwal J. Multiple intrahepatic pancreatic pseudocyst (MIHPPs): an overlooked and misdiagnosed entity. Gastroenterol Hepatol Bed Bench. 2019 Summer;12(3):263-266. PMID: 31528312; PMCID: PMC6668764.
- Demeusy A, Hosseini M, Sill AM, Cunningham SC. Intrahepatic pancreatic pseudocyst: A review of the world literature. World J Hepatol. 2016 Dec 18;8(35):1576-1583. doi: 10.4254/wjh.v8.i35.1576. PMID: 28050239; PMCID: PMC5165272.
- Baydar B, Cantürk F, Alper E, et al. Intrahepatic localization of pancreatic pseudocyst: A case report. Turk J Gastroenterol. 2013;24(5):447-449. doi:10.4318/tjg.2013.0805
- Topno N, Ghosh S, Baruah A. A Rare Case Report of Hepatic Subcapsular Pseudocyst of Pancreas. J Clin Diagn Res. 2016 Dec;10(12):PD18-PD19. doi: 10.7860/JCDR/2016/21843.9087. Epub 2016 Dec 1. PMID: 28208933; PMCID: PMC5296506.
- Tan JH, Chin W, Shaikh AL, Zheng S. Pancreatic pseudocyst: Dilemma of its recent management (Review). Exp Ther Med. 2021 Feb;21(2):159. doi: 10.3892/etm.2020.9590. Epub 2020 Dec 18. PMID: 33456526; PMCID: PMC7792492.
- Suggs P, NeCamp T, Carr JA. A Comparison of Endoscopic Versus Surgical Creation of a Cystogastrostomy to Drain Pancreatic Pseudocysts and Walled-Off Pancreatic Necrosis in 5500 Patients. Ann Surg Open. 2020 Nov 20;1(2):e024. doi: 10.1097/AS9.0000000000000024. PMID: 37637446; PMCID: PMC10455460.
- Chahal P, Baron TH, Topazian MD, Levy MJ. EUS-guided diagnosis and successful endoscopic transpapillary management of an intrahepatic pancreatic pseudocyst masquerading as a metastatic pancreatic adenocarcinoma (with videos). Gastrointest Endosc. 2009 Aug;70(2):393-6. doi: 10.1016/j.gie.2008.10.011. Epub 2009 Apr 25. PMID: 19394005.
- Jivani A, Shinde RK, Nagtode T, Vaidya K, Goel S. The Surgical Management of Pancreatic Pseudocysts: A Narrative Review. Cureus. 2024 Sep 10;16(9):e69055. doi: 10.7759/cureus.69055. PMID: 39391462; PMCID: PMC11465202.